Quick Links
Meet
the Commercial Team
Super User
Toggle
View
Scope of Service
Our entire business has been built to provide veterinarians and livestock producers the tools and information they need to “prevent the preventable” in order to keep their animals healthy and productive.
Diagnostic + Testing Services
We utilize advanced techniques and technology coupled with our extensive experience in diagnostics to work with veterinarians to ensure proper sampling, easy submission, quick turnaround time and accurate results. Our diagnostic services are aimed at isolating and identifying viral and bacterial pathogens and providing the information you need to select proper antibiotic therapy as well as the ability to include virus and bacteria isolate in a custom (autogenous) vaccine to prevent future disease.
Commercial Vaccine Production
For over 40 years we have been focused on developing novel, effective bovine vaccines that provide immunity against existing and emerging diseases that affect stocker and feeder cattle.
Custom Vaccine Manufacturing
When commercial vaccines are not providing you the protection you need against all of the viral and bacterial pathogens that are challenging your animals, we can manufacture a customized vaccine that fills the gaps your current vaccination protocol is leaving uncovered.
Bimeda Announces ‘Bimeda Biologicals Inc’ as Name for Acquired Vaccine Company
Bimeda Inc. is pleased to announce the formal renaming of acquired vaccine production, research and diagnostic company, Texas Vet Lab, Inc., as Bimeda Biologicals Inc. Both Texas Vet Lab, Inc. (TVL) and the VetBio, Inc. divisions of the business will henceforth operate under the new legal name of Bimeda Biologicals Inc.
Dave Cunningham, Bimeda's Chief Commercial Officer, commented, 'the renaming of the organization represents the first phase of our branding strategy and the continued integration of the TVL business, which is designed to see the company reach its potential both in domestic and export markets. The new name, Bimeda Biologicals Inc, respects the core identity of the business, while formally incorporating it into the Bimeda group of companies.'
Bimeda acquired the Texas-based biologicals business in December 2019, marking an important step for the company into the area of preventative medicines. At the time, Bimeda noted the company's significant potential for sustainable long-term growth and made a commitment to substantial investments to drive growth for both the existing vaccine portfolio and new product offerings.
For press queries, contact:
Dave Cunningham, Bimeda Chief Commercial Officer
Email:
Bimeda Welcomes Rey Armendariz
Bimeda® is pleased to announce Rey Armendariz in the new role of Regional Strategic Manager. Effective immediately, Rey will be an integral part of the food animal sales team and assist in the sales development in feedlots for both core Bimeda products and the newly acquired bovine biologicals commercial and custom vaccine lines. Rey will assist the food animal business unit by also facilitating the business development and creation of strategies for key strategic feedlot accounts throughout a three-state territory (Kansas, Nebraska and Colorado).
Rey has 21 years of experience in multiple segments of the animal health industry working for Fort Dodge Animal Health and most recently with Zoetis in the confined feeding area.
Bimeda is very proud to add Rey to our growing food animal business unit.
Bimeda Announces Acquisition of Texas Vet Lab, Inc
Bimeda is pleased to announce the acquisition of Texas Vet Lab, Inc (TVL). Established in 1977 and located in San Angelo, Texas, TVL specialises in bovine vaccine research and production, with a broad range of live and inactivated vaccines to protect against cattle respiratory diseases.
Company CEO Gavin Tierney, commented; 'the acquisition of Texas Vet Lab marks an important step for Bimeda into the arena of preventative medicine. Following on from the integration of TVL, Bimeda is able to offer our customers a broad suite of both preventative and curative treatment options, including vaccines, antimicrobials, anti-parasitics, hormones, and anti-inflammatories.'
Tierney added, 'TVL also boasts an on-site laboratory, which provides diagnostics for antibiotic sensitivity, as well as virus and bacteria isolation and identification. This will allow us to provide customers with a diagnostics-led approach to the treatment of their animals; ensuring maximum efficacy and rapid recovery from disease.'
The US cattle vaccine market has demonstrated strong annual growth over the past five years and this trend is set to continue in line with the global movement towards preventative medicine, and a more targeted use of antibiotics. Against this backdrop, the acquisition will serve as an important tool for Bimeda's long-term growth strategy in the US.
Dave Cunningham, Bimeda's Chief Commercial Officer for North America, commented; 'Texas Vet Lab is an exciting acquisition for Bimeda. With decades of experience in developing innovative vaccines, the TVL team brings a commitment to quality, innovation and serving the customer which perfectly aligns with Bimeda's own values. We see significant long-term growth potential for the business, and with our substantial planned investments in R&D, Sales and Marketing, we will drive growth for both the existing vaccine portfolio and new product offerings.'
Dr. Paul Lawrence
Tom Thompson
Rey Armendariz
David Christian
Reveal ATS
Not All Adjuvants are Created Equal
While adjuvants are a key ingredient in efficacious killed vaccines, not all adjuvants are created equal.
Reveal Antigen Transport System™ (ATS) is our proprietary adjuvant system that was specifically developed to improve the performance of our killed bacterial vaccines by creating a strong immune response while also reducing the harsh side effects often associated with other, more commonly used adjuvants.
It is incorporated into our PRO-BAC™ and MYCO-BAC® vaccines during the manufacturing process, so it comes ready-to-use and eliminates the hassle of chute-side mixing.
The Reveal ATS Advantage
Optimizes vaccine efficacy & stability
Attracts immune cells to the injection site to increase replication
Protects antigens from circulating anti-bodies
Enhances antigen presentation & processing for better immune response
Slowly releases the vaccine’s antigens for prolonged immune system exposure as well as the vaccine’s endotoxin to reduce harsh side effects often associated with vaccinating stressed cattle with gram negative vaccines
Bimeda USA
Bimeda® Biologicals
1702 North Bell Street,
San Angelo, Texas
76903
Phone: 1 800 284 8403

NO SMOKE & MIRRORS.
JUST SOLID SCIENCE.
Stimulator® vaccines were designed to stimulate a strong, protective immune response in stocker and feeder cattle against the five most common viral pathogens associated with Bovine Respiratory Disease (BRD):
- Infectious Bovine Rhinotracheitis (IBR)
- Bovine Viral Diarrhea Type 1 (BVD I)
- Bovine Viral Diarrhea Type 2 (BVD I)
- Bovine Respiratory Syncytial Virus (BRSV)
- Parainfluenza3 (PI3)
DOWNLOAD | STIMULATOR DETAILER
Why Choose Stimulator Vaccines?
![]() RELEVANT
RELEVANT
Stimulator vaccines were developed 10-20 years after most other commonly used straight MLV vaccines. As a result, an additional decade’s worth (or more) of insights and understanding were used to build Stimulator vaccines. We selected strains to maximize cross-protection against the most prevalent subtypes affecting your cattle.
![]() QUALITY
QUALITY
All viral antigens contained in Stimulator vaccines have been genetically tested to confirm stability and purity to ensure they have not mutated during the production process.
![]() SAFE
SAFE
Unlike most MLV’s for cattle, no fetal bovine serum is used at any stage of production of the viruses or the cells contained in Stimulator vaccines. This guarantees that no extraneous, unwanted bovine viruses are introduced from fetal bovine serum.
![]() EFFICACY
EFFICACY
Stimulates protective immunity so your cattle are better equipped to remain healthy and productive when faced with common viral challenges.
PROTECT YOUR CATTLE AND YOUR
BOTTOM LINE.
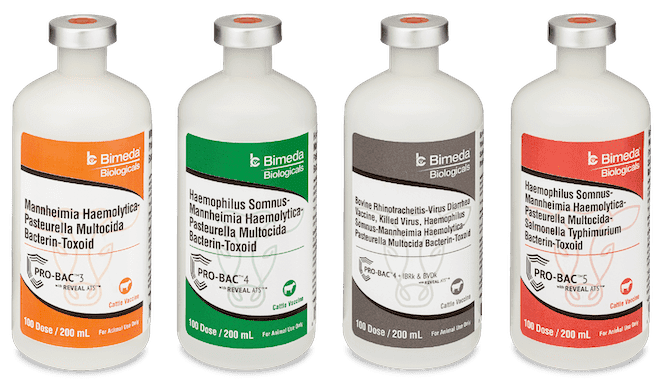
Our PRO-BAC™ vaccine line was developed specifically for stocker and feeder cattle and offers the most complete protection against five of the most common BRD-causing bacteria:
- Mannheimia haemolytica Type A1 (MhA1)
- Mannheimia haemolytica Type A6 (MhA6)
- Pasteurella multocida (Pm)
- Histophilus somni / Haemophilus somnus (Hs)
- Salmonella typhimurium (St)
No other vaccines available on the market can say the same.
All of the PRO-BAC vaccines are powered by our proprietary adjuvant Reveal ATS™. Learn more about the Reveal ATS Advantage
DOWNLOAD HIGH RISK CATTLE WITH PRO-BAC 4+IBR BVDk PRO-BAC - FEATURES, BENEFITS & COMPARISONS TO OTHER PRODUCTS PRO-BAC - OVERVIEW & IMPORTANCE OF PREVENTION PRO-BAC ADMINISTRATION GUIDE
Why Choose PRO‑BAC VACCINES?
![]() QUALITY BACTERIAL ANTIGENS
QUALITY BACTERIAL ANTIGENS
Our antigens are grown in specific conditions to maximize expression of all virulence factors, which optimizes post-injection antigen presentation in order to elicit a stronger immune response to better protect your cattle against the bacterial challenges they may face later on—whether that is on the ranch or in the feedlot.
![]() CONVENIENCE
CONVENIENCE
Available in both 50-dose and 100-dose bottles, PRO-BAC vaccines come ready-to-use requiring no chute-side mixing and are administered in a BQA-friendly 2.0 mL subcutaneous (SQ) dose in the side of the neck.
![]() VERSATILITY
VERSATILITY
Choose the right antigen combination to plug into your current vaccination protocol to fill the gaps other vaccines aren’t covering to set your calves up to perform on the ranch and in the feedlot.
![]() EFFICACY
EFFICACY
Our unique manufacturing process and proprietary adjuvant system (Reveal ATS™) results in vaccines that provide a high level of protection against the costliest BRD-causing bacteria without the harsh side effects often associated with the vaccination of mid- to high-risk cattle.
Learn more about the Reveal ATS Advantage
DEALING WITH HIGH PULL RATES, POOR TREATMENT RESPONSE AND SWOLLEN JOINTS IN RECENTLEY WEANED CALVES?
YOUR VACCINE PROTOCOL MAY BE MISSING A KEY PIECE.
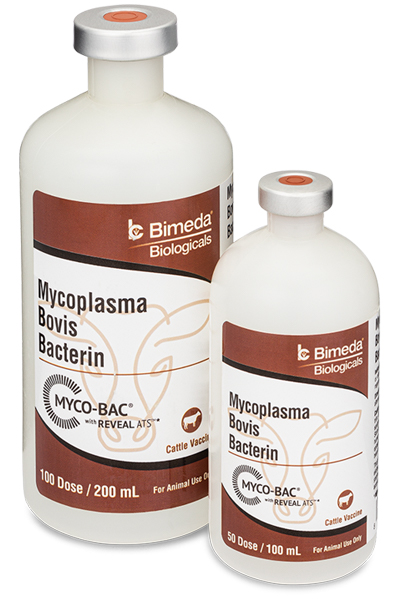
Mycoplasma bovis is one of the most prevalent of all the bacterial pathogens affecting young cattle during periods of elevated stress such as weaning and transportation. In general, the higher the “risk classification” of a particular group of cattle the greater the possibility of a Mycoplasma problem. Read
The typical “myco” infections begin to show up about 3 weeks after exposure and can cause respiratory disease as well as crippling arthritis. Due to it’s extremely small cell size and lack of a ridged cell wall, many antibiotics are rendered ineffective against M. bovis resulting in increased pull rates, poor treatment response and unusually high death rates.
MYCO-BAC® was the first fully licensed Mycoplasma bovis (also referred to as ‘Mycoplasma’ or ‘Myco’) vaccine approved by the USDA in 2002. It was originally developed by the team at Texas Vet Lab, Inc. following years of clinical observation and diagnostic/laboratory research as well as significant experience producing effective autogenous vaccines for feedlot and stocker veterinarians and consultants from across the country.

MYCO-BAC is powered by our proprietary adjuvant Reveal ATS™. Learn more about the Reveal ATS Advantage
DOWNLOAD | MYCO-BAC LABEL
The Benefits of Vaccinating with MYCO‑BAC
COST SAVINGS
When it comes to Mycoplasma bovis, prevention is almost always cheaper than treatment. Not only will a myco infection negatively impact cattle’s’ performance by keeping them off feed and water, effective treatment options are limited which often leads to high labor and drug expenses.
REDUCED MORBIDITY & MORTALITY
Field trials coupled with expansive experience and understanding of the disease support that giving MYCO-BAC, according to the labeled 3-dose regiment administered on days 0, 7 and 14, will reduce morbidity and mortality as well as increase treatment response success in vaccinated cattle.
EFFECTIVE
Our unique manufacturing process combines quality bacterial antigen with our proprietary adjuvant system (Reveal ATS™) to create a strong, specific immune response to prevent and control respiratory disease due to M. bovis.
Learn more about the Reveal ATS™ Advantage

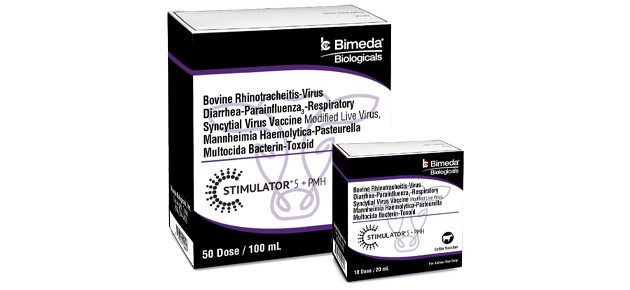
PREVENTING THE PREVENTABLE
WITH AN EFFECTIVE COMBINATION.
An effective combination vaccine that helps protect cattle against some of the most common—and costly—viral and bacterial pathogens associated with Bovine Respiratory Disease (BRD).
Available Sizes
10 dose, 50 dose
DOWNLOAD | STIMULATOR 5 + PMH DETAILER










